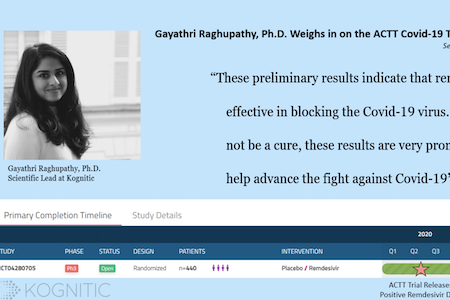
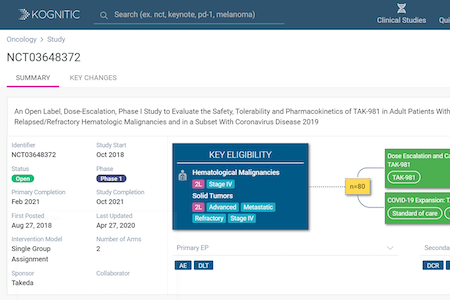
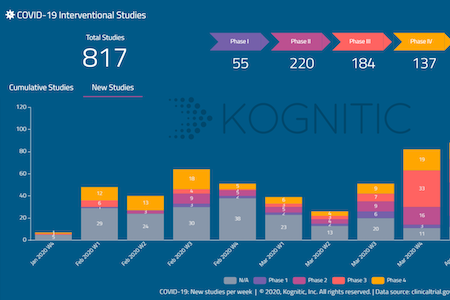
ACTT Trial Results Indicate Remdesivir’s Efficacy Against Covid-19
May 1, 2020
What you must know about ADCs
February 23, 2021
We were deeply saddened to learn of Chadwick Boseman’s passing after a four year battle with colorectal cancer. Chadwick inspired and entertained millions through his portrayal of many iconic figures: Jackie Robinson, James Brown, Thurgood Marshall, & most notably his barrier-breaking role as King T’Challa in Marvel’s Black Panther. In the spirit of T’Challa’s quote, “We all know the truth: more connects us than separates us,” we acknowledge how we are unified in the war on cancer and the fight for a cure.
The loss of Boseman is sparking the much-needed awareness about colon cancer, especially in the younger population (under age 50). Young-onset colorectal cancer is on the rise; awareness and education about early diagnosis are crucial to tackling this disease. A recent survey conducted by the Colorectal Cancer Alliance, shows that late diagnosis or misdiagnosis is quite common in the younger patient population. Moreover, the lack of knowledge or proper channels of communication on clinical trial opportunities, biomarker testing, or the impact of treatment on fertility and lifestyle, hinders shared decision making and may leave patients unaware of all possible options.
Given Kognitic’s expertise in drug development and clinical trial intelligence, we would like to take a moment to share some basic facts about clinical trials and biomarkers that can hopefully serve the purpose of empowering patients with knowledge.
General information about clinical trials and biomarkers
Clinical trial
What is a clinical trial?
Clinical trials test if new drugs, devices, or already approved drugs are effective in treating a disease condition. In most situations, clinical trials are conducted to identify a new treatment that is better than an already existing standard treatment. Every prescribed disease treatment that exists today has gone through the clinical trial process to be approved for prescribing.
The journey of a drug begins in a laboratory; once the drug is shown to be effective in animal (pre-clinical) studies, the company or institution seeks permission from authorities (FDA) to study the drug in humans and execute a rigorously planned and regulated clinical trial.
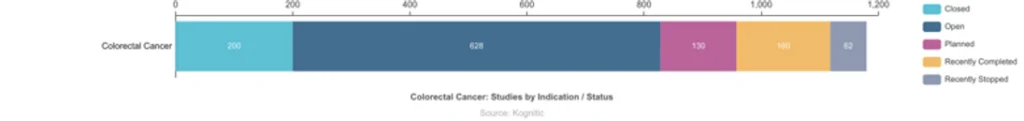
Numerous clinical trials are running worldwide for colon cancer; there are 1180 trials worldwide, of which 628 are currently open to patient recruitment. Navigating through these many clinical trials can be challenging, but several resources are available online to help screen through trials, and patients are encouraged to discuss clinical trial options with their physicians.
Biomarker
What is a biomarker?
Biomarkers are indicators of the presence or absence of disease or the effectiveness of treatment. Molecular biomarkers include tissue, blood, DNA, urine, stool, or other bodily fluids.
Biomarker spotlight: MSI-H
Microsatellite Instability – High (MSI-H) is found in about 15% of CRC tumors. MSI-H tumors have excessive mutations in a specific set of DNA called microsatellites caused due to a defect in the DNA repair process (which acts as a spell-check on DNA). MSI-H can be tested through DNA samples.
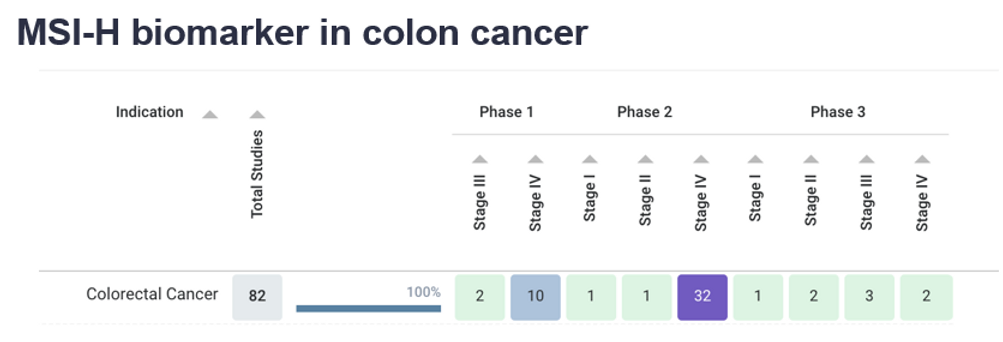
The heatmap above displays the colon cancer clinical trials treating patients with MSI-H. The trials are broken down by both the trial phase (Phase 1, 2, or 3) and stage of the cancer being treated (Stage I, II, III, and IV).
Useful sources:
https://fightcolorectalcancer.org/
https://www.ccalliance.org/
https://www.cancer.gov/types/colorectal
Reference: