ESMO 2023 Emerging Trends: A Preview of Abstracts
October 19, 2023
ASH 2023 Rapid Insights: Acute Myeloid Leukemia
January 18, 2024
ctDNA in Oncology:
Current Landscape and Future Potential
by Jyoti Pant and Hunter Moody
ctDNA in Oncology:
Current Landscape and Future Potential
by Jyoti Pant and Hunter Moody
Circulating tumor DNA (ctDNA) is a type of cell-free DNA (cfDNA) specific to tumor-derived DNA. It can be detected in blood samples and offers a non-invasive alternative (often referred to as “liquid biopsy”) for monitoring tumor burden and evolution, identifying targetable mutations, and predicting cancer recurrence (1). While it was first validated as a biomarker in metastatic colorectal cancer to detect KRAS and BRAF mutations (2), ctDNA has since been used to characterize various other cancers, including non-small cell lung cancer (NSCLC), breast cancer, and head and neck cancer (3, 4, 5, 6).
Despite still being a relatively new biomarker, ctDNA has the potential to revolutionize the way we diagnose, monitor, and treat cancer. Its emergence has led to a growing body of evidence to support its use, and further efforts are underway to fully understand its role(s) in oncology. In fact, in May 2022, the US Food and Drug Administration (FDA) released draft guidance on the use of ctDNA as a biomarker in clinical trials for early-stage solid tumors (7). The document outlines potential uses for ctDNA as can be seen below:
Patient selection: ctDNA has the potential to be used to identify patients with genetic/epigenetic alterations who may benefit from targeted drugs. Clinical trials may also use ctDNA as a stratification factor (e.g., ctDNA positive vs. ctDNA negative).
Marker of Minimal Residual Disease (MRD): ctDNA can also be used as a marker of MRD. This could allow clinical trials to enroll/stratify patients based on disease risk via ctDNA status. Patients with ctDNA-positive status may be deemed as having a “higher-risk” disease with an increased risk of recurrence.
Measure of response: ctDNA could be used to support trials by aiding in signaling drug activity.
Early endpoint: changes in ctDNA in response to a drug also have the potential to be used as an early endpoint for early-stage solid tumor trials.
Further, additional uses for ctDNA are also being explored (1). Notably, ctDNA has garnered significant interest in cancer screening and detection. The ability to accurately screen and detect cancer at an early stage with ctDNA would be an enormous feat and would have the potential to significantly improve patient outcomes.
To assess the current landscape of ctDNA in oncology, we analyzed over 58,000 active clinical trials and identified 687 (1.18%) trials using ctDNA. The majority of ctDNA trials are Phase II (71%), followed by Phase I (16%) and Phase III (12%). Within these trials, we identified 399 pipeline drugs and 184 targets. Furthermore, we saw that 84% of ctDNA trials are using ctDNA to assess treatment efficacy, compared to 16% that are using ctDNA for patient selection.
We also looked at the annual trend of ctDNA trials over the past 10 years (2014 to 2023). It can be seen that the number of new ctDNA trials annually has trended upward during this time span (Figure 1).
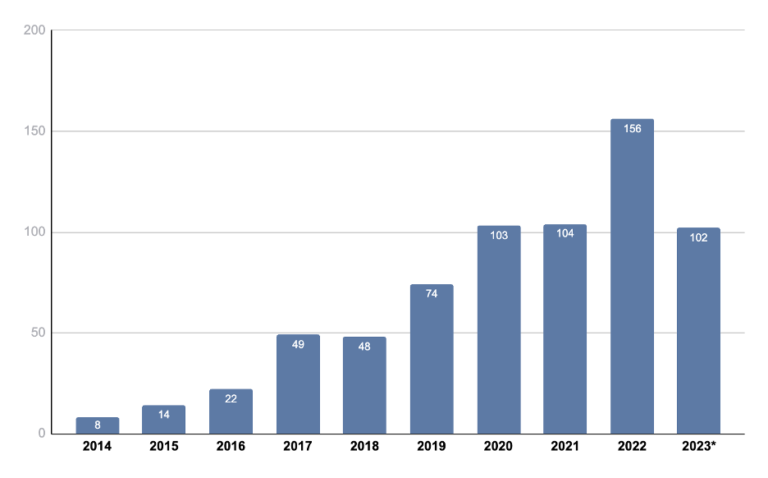
Figure 1: Annual study trend of clinical trials with ctDNA usage over the past 10 years.
*Data through September 14, 2023. Source: Kognitic
Looking at the number of clinical trials using ctDNA by company, AstraZeneca and Roche lead the way in this category, with Merck rounding out the top 3 (Figure 2).
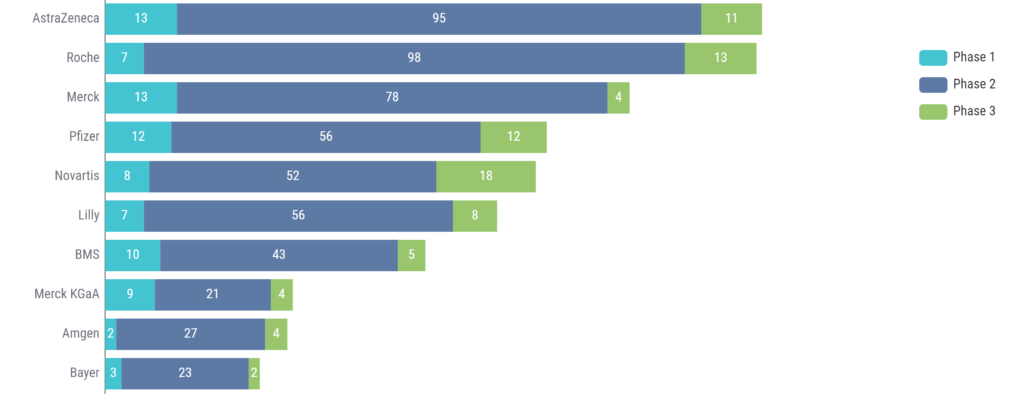
Figure 2: Top 10 companies using ctDNA in clinical trials. Source: Kognitic
Additional trends for ctDNA trials can be seen by analyzing the top drugs being used in these trials. We can see that four of the top drugs (based on number of trials) are immune checkpoint inhibitors (ICIs), and osimertinib is the only non-ICI to break into the top 3 (Figure 3).
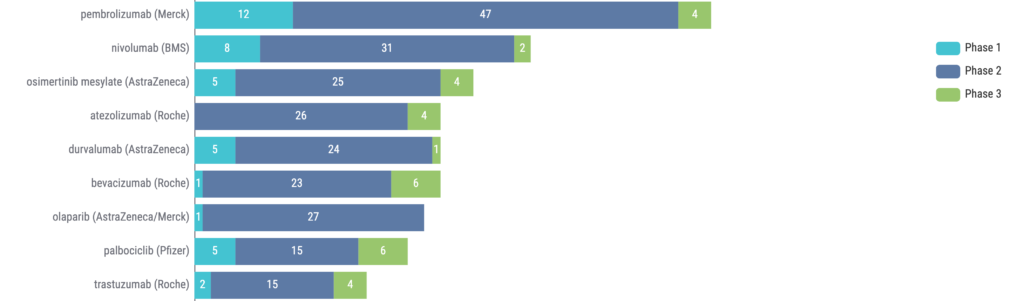
Figure 3: Top drugs (company) in clinical trials using ctDNA. Source: Kognitic
It is also evident that ctDNA is being investigated across a wide range of indications. NSCLC sits atop the list, with breast cancer and colorectal cancer in the second and third spots, respectively (Figure 4).
Moreover, the use of ctDNA is also being explored in hematologic malignancies (8). For example, the potential role of ctDNA as a marker for MRD is being evaluated in hematologic malignancies such as multiple myeloma and diffuse large B-cell lymphoma.
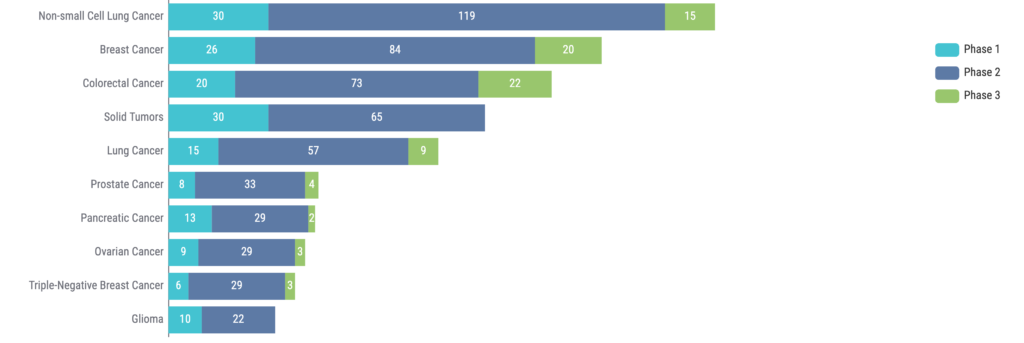
Figure 4: Top 10 indications for clinical trials using ctDNA. Source: Kognitic
Lastly, we analyzed new study trends over the past 12 months (November 2022 to November 2023). We identified 8,859 new oncology clinical trials, 161 (1.82%) of which were ctDNA trials. The new study trends for ctDNA trials are shown in Figure 5.
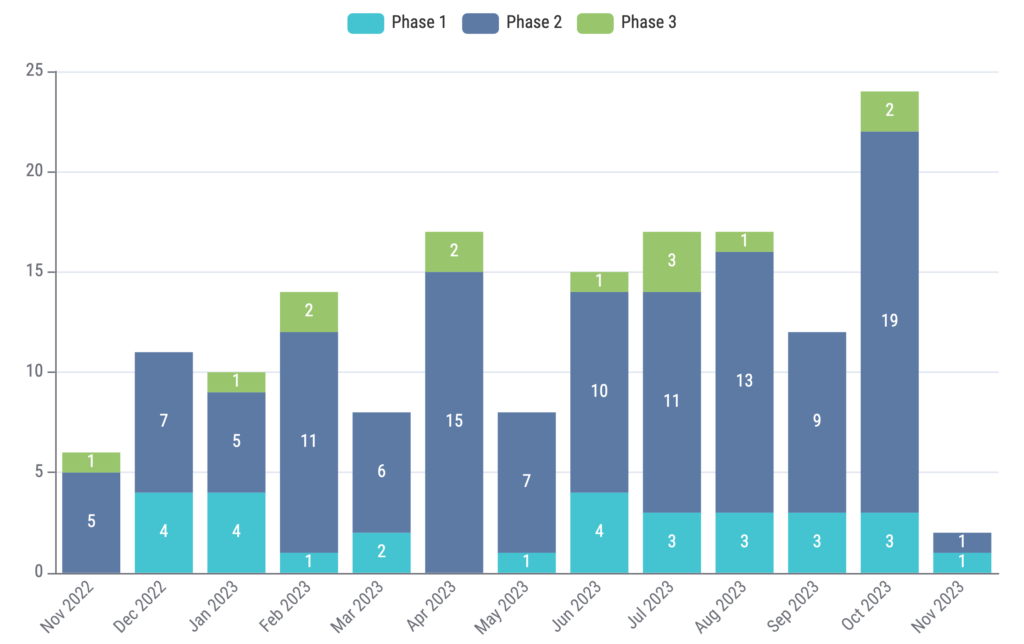
Figure 5: New study trend of clinical trials with ctDNA usage over the past 12 months. Source: Kognitic
Conclusion
Our analysis shows the robust and dynamic landscape of ctDNA within oncology. With a plethora of potential uses, ctDNA is a biomarker that is sure to have a growing impact in the coming years. From prognostic biomarkers to tools for early detection, ctDNA is able to provide us with a wealth of information. It is clear that this is an area to watch as new data continues to emerge. Although only a small percentage of oncology trials (~1.8%) currently implement ctDNA, the gradually increasing annual trend (Figure 5) in ctDNA trials shows that ctDNA will continue to gain traction and capture trial share within the oncology landscape.
References
-
Fiala C, Diamandis EP. Utility of circulating tumor DNA in cancer diagnostics with emphasis on early detection. BMC Med. 2018;16(1):166. Published 2018 Oct 2.
-
Thierry AR, Mouliere F, El Messaoudi S, Mollevi C, Lopez-Crapez E, Rolet F, Gillet B, Gongora C, Dechelotte P, Robert B, Del Rio M, Lamy PJ, Bibeau F, Nouaille M, Loriot V, Jarrousse AS, Molina F, Mathonnet M, Pezet D, Ychou M. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med. 2014 Apr;20(4):430-5. doi: 10.1038/nm.3511. Epub 2014 Mar 23. PMID: 24658074.
-
Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, Liu J, Pantel K. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012 Oct 15;18(20):5701-10. doi: 10.1158/1078-0432.CCR-12-1587. Epub 2012 Aug 20. PMID: 22908097.
-
Xia L, Mei J, Kang R, Deng S, Chen Y, Yang Y, Feng G, Deng Y, Gan F, Lin Y, Pu Q, Ma L, Lin F, Yuan Y, Hu Y, Guo C, Liao H, Liu C, Zhu Y, Wang W, Liu Z, Xu Y, Li K, Li C, Li Q, He J, Chen W, Zhang X, Kou Y, Wang Y, Wu Z, Che G, Chen L, Liu L. Perioperative ctDNA-Based Molecular Residual Disease Detection for Non-Small Cell Lung Cancer: A Prospective Multicenter Cohort Study (LUNGCA-1). Clin Cancer Res. 2022 Aug 2;28(15):3308-3317. doi: 10.1158/1078-0432.CCR-21-3044. PMID: 34844976.
-
Aulakh SS, Silverman DA, Young K,Dennis SK, Birkeland AC. The Promise of Circulating Tumor DNA in Head and Neck Cancer. Cancers (Basel). 2022 Jun 16;14(12):2968. doi: 10.3390/cancers14122968. PMID: 35740633; PMCID: PMC9221491.
-
www.clinicaltrials.gov
-
FDA 2022. https://www.fda.gov/media/158072/download
-
Talotta D, Almasri M, Cosentino C, Gaidano G, Moia R. Liquid biopsy in hematological malignancies: current and future applications. Front Oncol. 2023;13:1164517. Published 2023 Apr 20.
Copyright© 2023 Kognitic, inc. All rights reserved.*