COVID Vaccines, what’s new: an update on the latest clinical trials that are underway.
May 24, 2021
Exploring Boundless Potential: AI Revolutionizes Oncology at ASCO 2023
June 23, 2023CURRENT LANDSCAPE OF CELL THERAPY CLINICAL TRIALS
The first cell therapy (Provenge) to treat cancer got FDA approval in 2010. Following the approval of Provenge, we have six marketed chimeric antigen receptor T-cell (CAR-T) therapies (Table 1), and we eagerly anticipate the development of additional cell therapies in the future.
Here we have tried to understand the cell therapy landscape and its future outlook. Our major findings are: a) cell therapy trials are unequally distributed across different phases with more trials in Phase I and II; b. The majority of cell therapy trials are treating blood cancer; c. there is a gradual growth in solid tumor trials in recent years; and most cell therapy trials are targeting CD19.
| Name | Year of Approval |
Type | Manufacturer | Description |
| PROVENGE | 2010 | Autologous | Dendreon Corp. | Activated antigen-presenting cells targeting prostatic acid phosphatase to treat certain types of prostate cancer. |
| KYMRIAH | 2017 | Autologous | Novartis Pharmaceuticals Corporation | Engineered CAR T cells to attack CD19-expressing tumor cells to treat patients with certain kinds of relapsed or refractory follicular lymphoma. |
| YESCARTA | 2017 | Autologous | Kite Pharma, Inc. | Engineered CAR T cells targeting CD19-expressing tumor cells to treat patients with certain kinds of relapsed or refractory large B-cell lymphoma. |
| TECARTUS | 2020 | Autologous | Kite Pharma, Inc. | Engineered CAR T cells targeting CD19-expressing tumor cells to treat patients with certain kinds of relapsed or refractory mantle cell lymphoma. |
| ABECMA | 2021 | Autologous | Bristol-Myers Squibb Company | Engineered CAR T cells targeting BCMA to treat multiple myeloma. |
| BREYANZI | 2021 | Autologous | Bristol-Myers Squibb Company | Engineered CAR T cells targeting CD19-expressing tumor cells to treat patients with certain kinds of large B-cell lymphoma. |
| CARVYKTI | 2022 | Autologous | Johnson & Johnson | Engineered CAR T cells targeting BCMA-expressing tumor cells to treat patients with certain kinds of relapsed or refractory multiple myeloma. |
Table 1: FDA-approved oncology cell therapy (1)
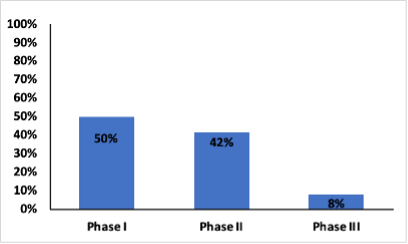
The majority of clinical trials in cell therapy are in Phase I and Phase II
There are a total of 1823 cell therapy clinical trials, 141 of these did not have phase-specific information. Of the remaining 1680 trials, 67% are open, active trials, 16% are planned trials, and 17% are closed. Phase I has the highest number of trials accounting for 46% of the trials, followed by 39% in Phase II and only 8% in Phase III (Figure 1).
For more information on the trial designs, status, and updates, sign up for a free demo.
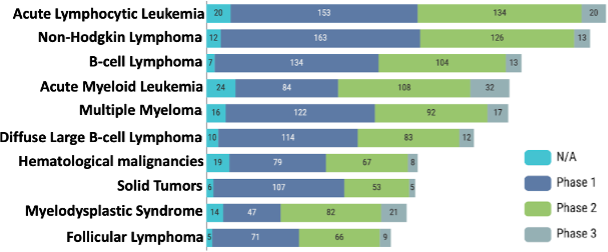
Gradual growth in cell therapy trials targeting solid tumors
Of the total 1819 trials in the cell therapy subclass, 60% (1106) of the trials are for blood cancer indications such as leukemia, lymphoma, and myeloma, and 40% (713) of the trials are for various solid tumors. Among the top 10 indications for cell therapy trials, 9 out of 10 are blood cancer. The indication with the highest number of clinical trials is acute lymphocytic leukemia, with 328 trials, followed by Non-Hodgkin lymphoma, B-cell lymphoma, Acute Myeloid Leukemia, and Multiple Myeloma, the top five indications (Figure 2).
Treating solid tumors using cell therapy has been challenging for various reasons, such as insufficient recruitment and penetration of solid tumors by cytotoxic T cells, immunosuppression in the solid tumor microenvironment, and lack of efficient solid tumors, to name a few. However, with advancements in target identification and gene and cell therapies, there has been a gradual growth in research investigating cell therapies for treating solid tumors, as observed by an increasing number of clinical trials every year (Figure 3).
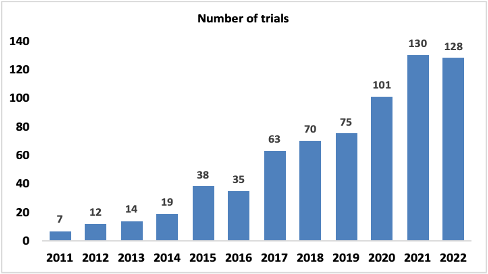
Source: https://kognitic.com. For more information on trial dates, sign up for a free demo.
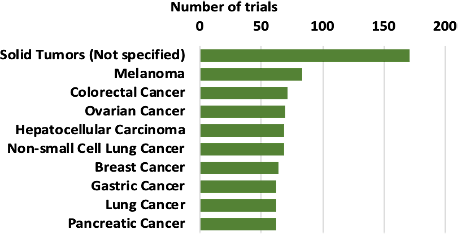
Most cell therapy clinical trials treating solid tumors are treating solid tumors not specified in the trials. Melanoma, colorectal, and ovarian cancer are other solid tumors with the highest number of clinical trials (Figure 4).
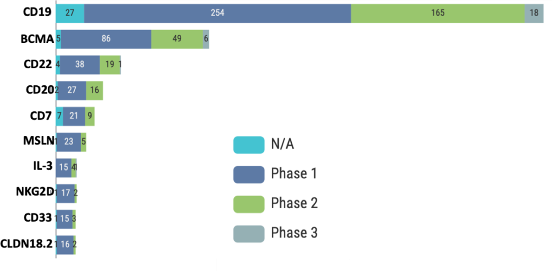
CD19 is the most common target for cancer cell therapy trials
For more information on the target, drugs, and modality, sign up for a free demo.
Most trials in cell therapy target CD19, a type I transmembrane glycoprotein on the surface of B- cell lymphocytes. Four of seven FDA-approved CAR-T cells target CD-19 and treat hematological malignancies (Table 1). This surface antigen plays a critical role in the immune response by modulating B-cell receptor-dependent and independent signaling. It is expressed on the surface of normal B-cells. It is expressed at average to high levels in many B cell malignancies, such as adult acute lymphoblastic leukemia, B cell lymphomas, and B cell leukemia. Therefore, it is an attractive target for many hematological malignancies (2).
The other top targets are BCMA, CD22, CD20, CD7, MSLN, IL-3, NKG2D, CD33, and CLDN18.2 (Figure 5). The high number of clinical trials targeting CD19, BCMA, CD20, and other proteins highlights the significant research in cell therapy treating hematological malignancies such as ALL and NHL by targeting CD19 and other B cell antigens. Additionally, studies with targets such as MSLN, IL-3, and CLDN18.2 highlight the potential for cell therapy in treating solid tumors.
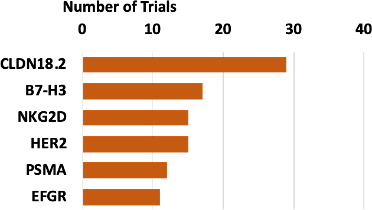
The top targets for solid tumor cell therapy clinical trials are CLDN18.2, B7-H3, NKG2D, HER2, PSMA, and EFGR (Figure 6). Claudin 18.2 is a tight junction protein exclusively expressed in differentiated gastric mucosal membrane epithelial cells that is overexpressed in several cancers, such as pancreatic cancer, esophageal cancer, ovarian adenocarcinoma, and lung cancers, and hence is an attractive target candidate to treat various cancers (3).
For more information on the target, drugs, and modality, sign up for a free demo.
Conclusion
Our analysis shows that cell therapy clinical trials are emerging with new trials registered in the early phases. Most of this research is focused on treating blood cancers and hematological malignancies targeting CD19, BCMA, CD22, etc. Although solid tumors are difficult to treat with cell therapy, with recent advancements in cell and gene therapy, there is a rise in the number of trials in treating solid tumors using cell therapy, giving us hope for new therapeutics to treat both solid and liquid cancers in the future.
References:
- https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-productsproducts/approved-cellular-and-gene-therapy-products
- Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis, and therapy. Exp Hematol Oncol. 2012 Nov 29;1(1):36. doi: 10.1186/2162-3619-1-36. PMID: 23210908; PMCID: PMC3520838.
- Cao W, Xing H, Li Y, Tian W, Song Y, Jiang Z, Yu J. Claudin18.2 is a novel molecular biomarker for tumor-targeted immunotherapy. Biomark Res. 2022 May 31;10(1):38. doi: 10.1186/s40364-022-00385-1. PMID: 35642043; PMCID: PMC9153115.
- https://kognitic.com
- https://clinicaltrials.gov
For more information, email info@kognitic.com
Copyright© 2023 Kognitic, inc. All rights reserved.*