ASH 2023 Rapid Insights: Acute Myeloid Leukemia
January 18, 2024
Beyond CI Teams: A Strategic Intelligence Platform Guide for Pharma R&D, BD, and Biotech Leaders
December 23, 2024
Oncology Clinical Trials:
Outlook and Emerging Trials
by Hunter Moody, Gayathri Devi, Aparna Jadhav
Oncology Clinical Trials: Outlook and Emerging Trials
by Hunter Moody, Gayathri Devi, Aparna Jadhav
Introduction
Kognitic uses Artificial Intelligence/Machine Learning to transform pharmaceutical intelligence into forward-looking insights across the value chain.
In this report, Kognitic provides insight based on the clinical trial landscape of 2023. This AI-powered report highlights key data and trends from 2023, unlocks potential trends moving forward, and puts a spotlight on antibody-drug conjugates (ADCs).
In total, Kognitic analyzed over 18,500 new trials for the creation of this report. More information on the Kognitic platform can be found at https://kognitic.com or by emailing info@kognitic.com.
Predominately Phase II Trials
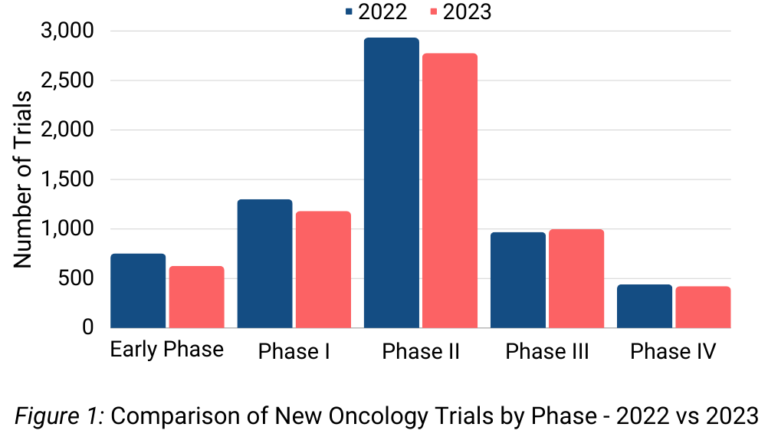
We analyzed trials in 2022 and 2023, categorizing them based on their designated study phase. In 2022, the total number of trials amounted to 9567, with 32% (3168) lacking specific phase information. The total number of trials in 2023 saw a 6% reduction, totaling 8953; of these, 33% (2946) trials had no phase information. Trials lacking phase information were excluded from our analysis.
Among the remaining 6399 trials in 2022 and 6007 trials in 2023, Phase II trials constituted the highest proportion for each year, accounting for 45% of 2022 trials and 46% of 2023 trials.
Notably, there was a marginal decline observed in the number of trials across Early Phase, Phase I, Phase II, and Phase IV from 2022 to 2023. However, there was a slight increase in the number of Phase III trials in 2023 compared to 2022 (Figure 1).
Gastrointestinal Cancer Persists as a Leading Oncology Indication
Oncology clinical trials were distributed across 20 indications. The indication being investigated in the highest number of trials was gastrointestinal cancer, accounting for ~24% of indications in 2022 trials and ~23% in 2023 trials (Figure 2). The top 5 indications for 2023 were gastrointestinal cancer, lung cancer, breast cancer, non-Hodgkin lymphoma, and gynecologic cancer.
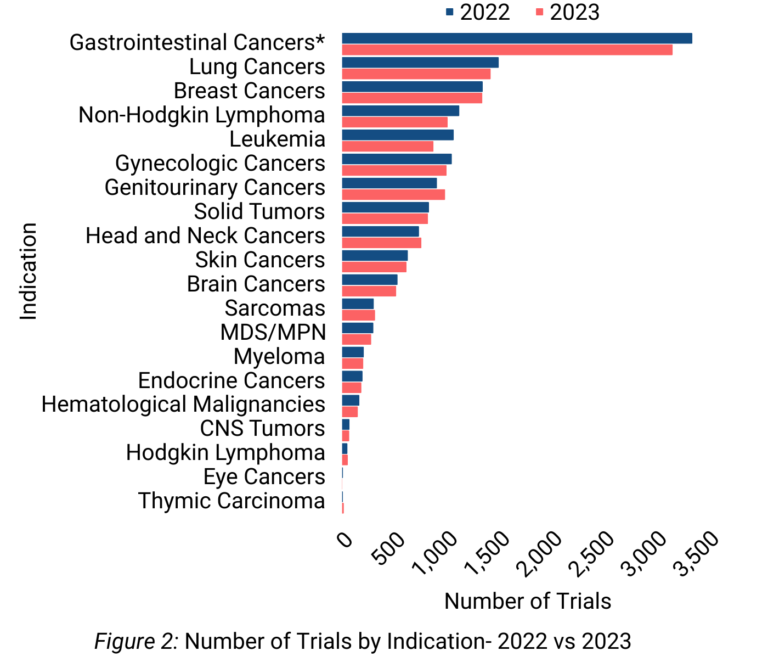
Recent reports indicate that gastrointestinal cancers such as colorectal, pancreatic, and liver cancers are exhibiting increased incidence rates, even amongst younger demographic groups. (1,2)
Outcomes for many gastrointestinal malignancies continue to be poor, with five-year survival rates below 30% for cancers like esophageal, liver, and pancreatic. This combination of rising gastrointestinal cancer burden and persistently low survival underscores a high unmet need. Consequently, research efforts are expanding to develop improved treatment options, as evidenced by the growing number of clinical trials focused on investigating novel therapies for gastrointestinal cancers. (1,2)
Lung cancer continues to be the next major focus in oncology, with trials accounting for approximately half the number of gastrointestinal cancer trials. The persistent high burden of lung cancer coupled with the opportunities to further improve therapeutic strategies, such as combination therapy, contributes to lung cancer representing the second largest focus for cancer clinical trials. (3,4)
Dominant Modalities: Small Molecules and Monoclonal Antibodies, and the Emergence of Antibody-Drug Conjugates (ADCs)
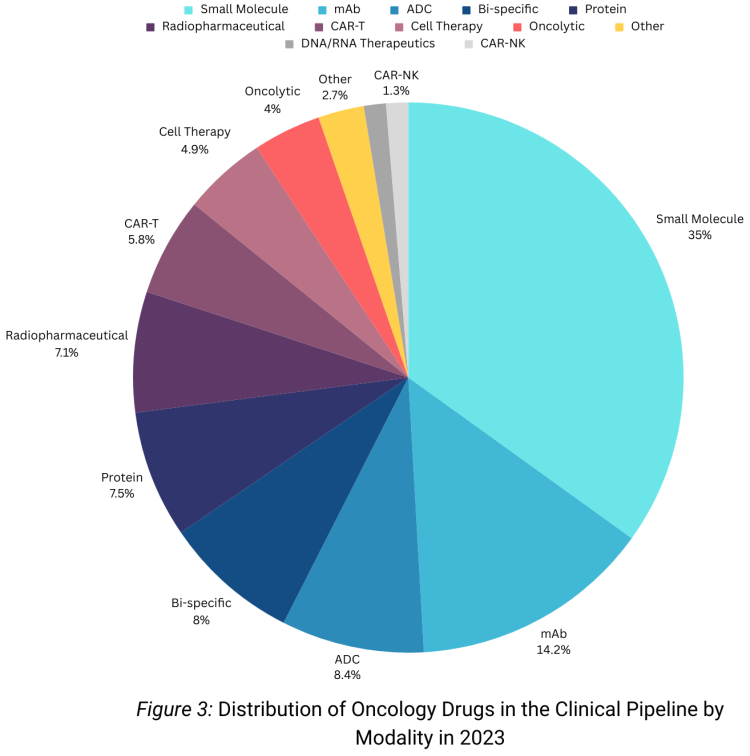
Regarding the distribution of drugs by modalities (Figure 3), small molecules and monoclonal antibodies remain prominent, mirroring patterns observed in recent years. Of all the novel therapies undergoing clinical trials, 34% are small molecules, and 13.6% are monoclonal antibodies.
While small molecules and monoclonal antibodies maintain a significant presence in clinical trials, there is a noticeable emergence of a third major therapeutic category—ADCs, with an 8.9% count. A significant count is seen for different types of cell therapies, and radiopharma therapeutics. It is worth highlighting that there has been a decrease in new cell therapies compared to 2022.
PD-1 trials continue to lead along with surging interest in promising targets
We analyzed new drugs entering clinical trials in 2023 and compiled a list of the top molecular targets. Our analysis indicates that PD-1 remains the most common therapeutic target in oncology clinical trials (Figure 4).
Despite the sustained interest in traditional checkpoint inhibitors, there is a noticeable shift towards targeted therapies.
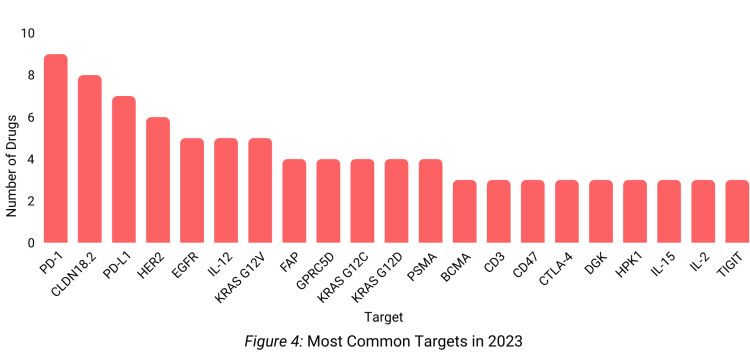
Oncology drug development is expanding beyond well-known targets, as evidenced by the increasing number of compounds aimed at newer antigens like CLDN18.2, GPRC5D, and KRAS.
In keeping with the movement towards precision oncology, 2023 witnessed the rise of new therapeutic targets like DGK (Diacylglycerol kinases), enabling greater selectivity. DGK inhibitors are being explored by companies like Bayer, BMS, Astellas, and BeiGene.
Radiotracers and radiotherapeutics selectively targeting the tumor-associated proteins, prostate- specific membrane antigen (PSMA) and fibroblast activation protein (FAP), continue to garner interest in cancer detection and treatment.
Rising Interest in Dual-targeting Therapeutics
The analysis revealed an emerging trend of dual- targeting drugs, such as bi-specific CAR-T cell therapy or bi-specific ADCs. For example, currently, there are 3 bi-specific ADC drugs in development, that target combinations of tumor antigens including EGFRxHER3, HER2xHER3, and EGFRxcMET. These 3 bi-specific ADC candidates contributed 7 new phase I trials in 2023 out of a total of 18 ongoing trials.
Additionally, with growing interest in bi-specific T cell engagers (BiTEs), we saw new bi-specific CAR-T cell therapies engaging dual targets like GPRC5DxBCMA and CD20xCD19. These combined modalities aid in harnessing dual anti- cancer mechanisms by engaging multiple tumor antigens or targets simultaneously.
ADCs CONTINUE TO STAY AT THE FOREFRONT
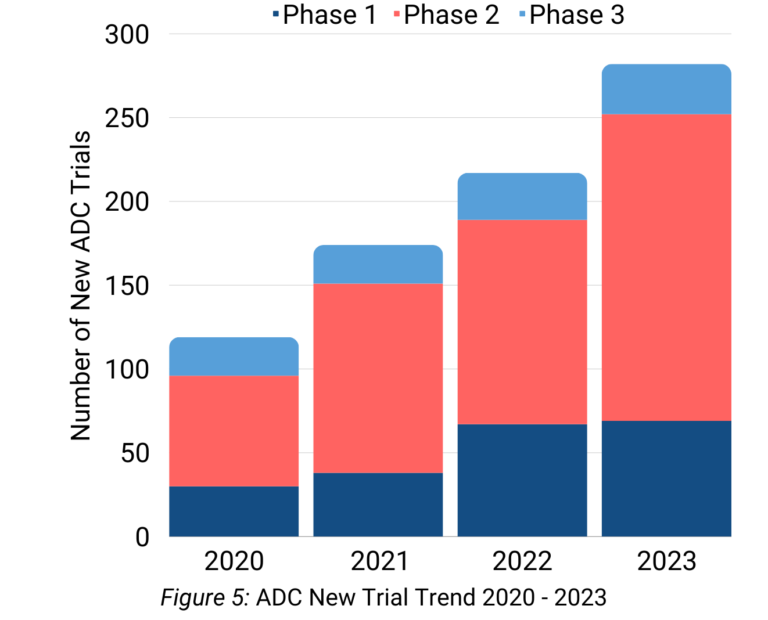
Antibody-drug conjugates (ADCs) continued to strengthen their position within the oncology space in 2023. Although no new ADCs were approved in 2023, new trials investigating ADCs continued to trend upwards (Figure 5). There were nearly 300 new ADC trials in 2023, with the majority of these trials being phase 2 trials.
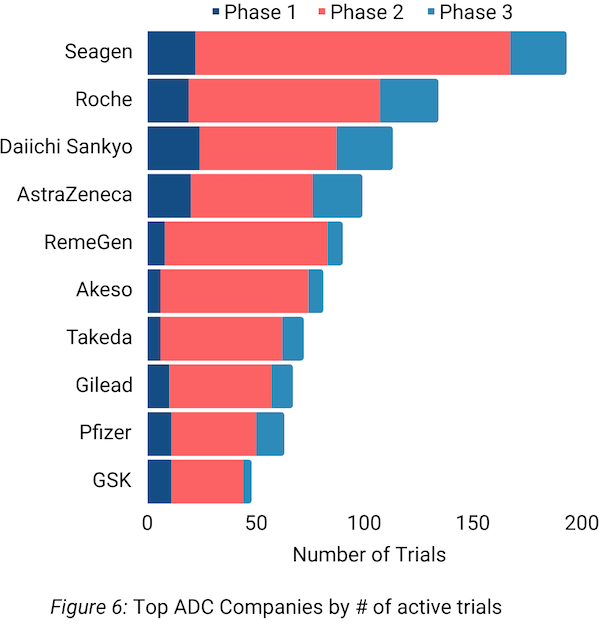
The current leader (by number of active trials; Figure 6) in the ADC space, Seagen (now part of Pfizer), saw 59 oncology trials start that included one of their ADCs in 2023. While nearly all of Seagen’s ADCs use MMAE as the payload, their targets include: HER2, Nectin-4, CD30, tissue factor, ITGB6, and CEACAM5.
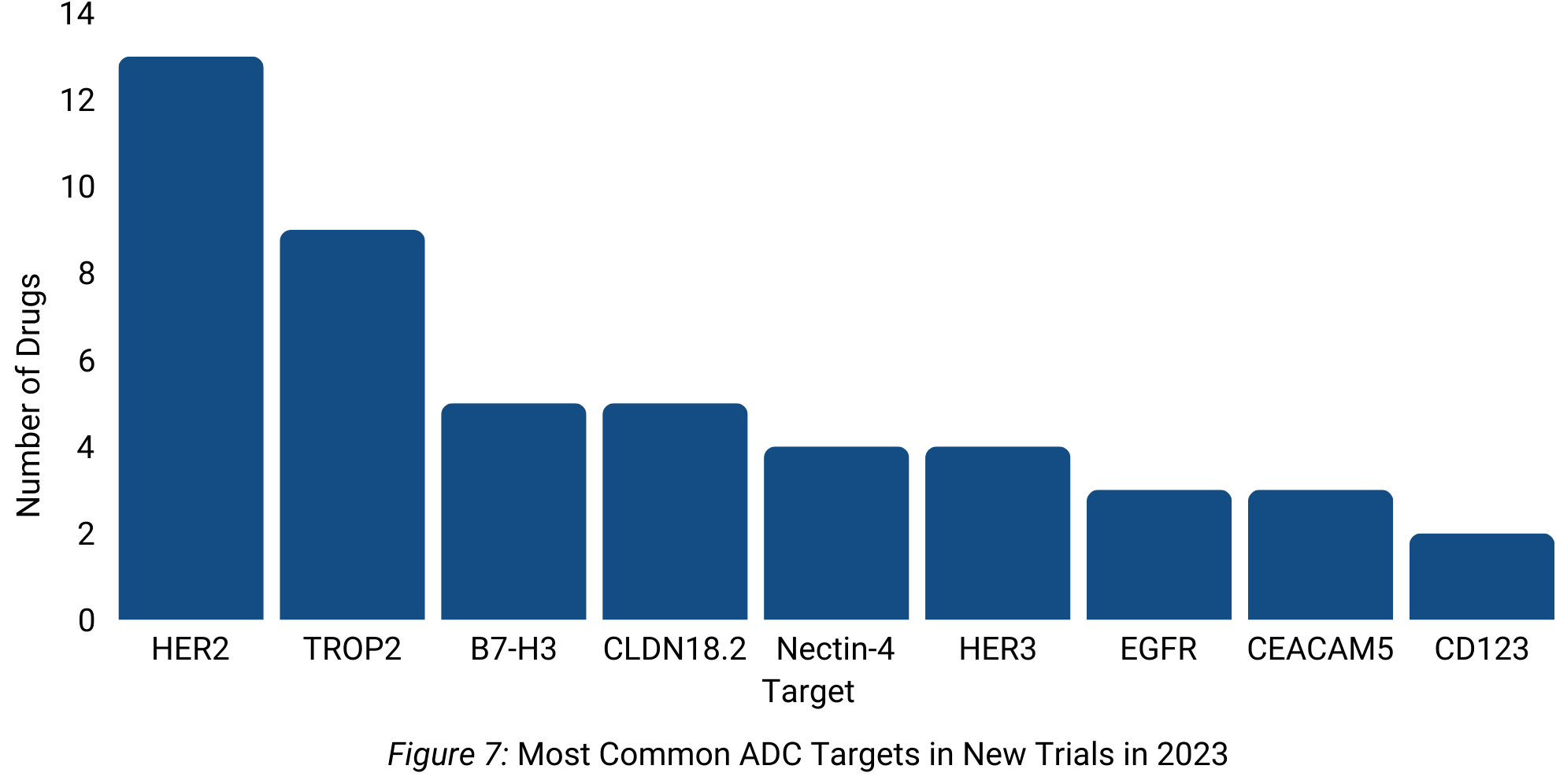
Regarding targets, HER2 remains the most popular, with more than 10 HER2-directed ADCs starting a new trial in 2023 (Figure 7).
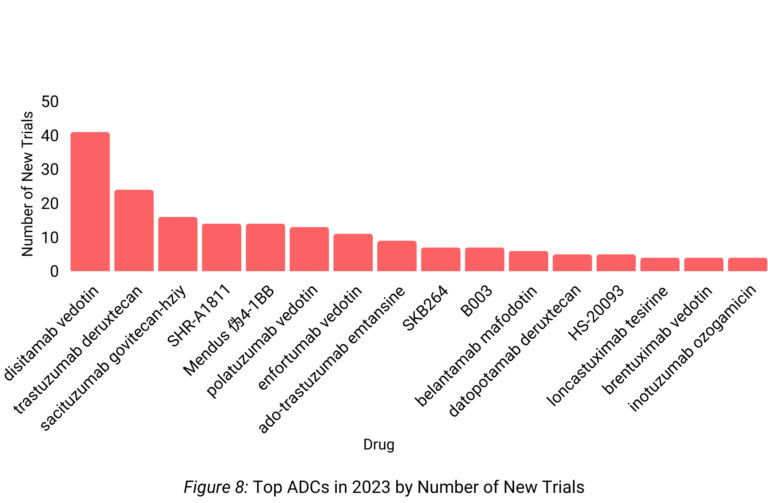
Disitamab vedotin led the way for ADCs in terms of new trials for 2023 (Figure 8). Disitimab vedotin saw an impressive 41 new clinical trials.
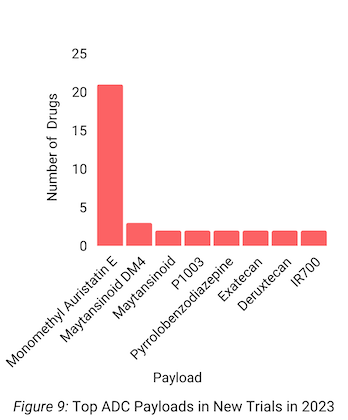
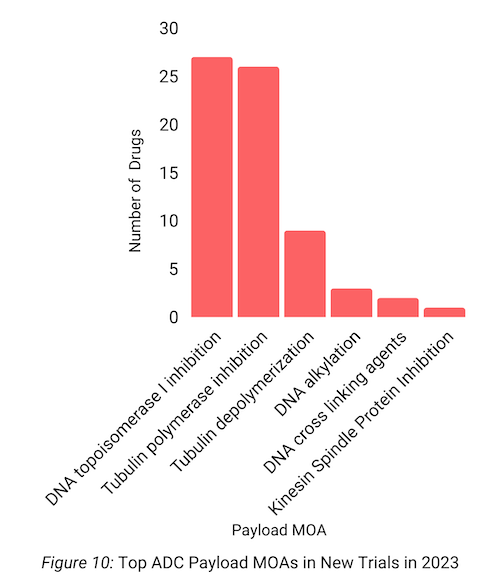
Furthermore, MMAE and DNA topoisomerase I inhibition were the most common ADC payloads and payload mechanisms of action for trials started in 2023, respectively (Figures 9-10).
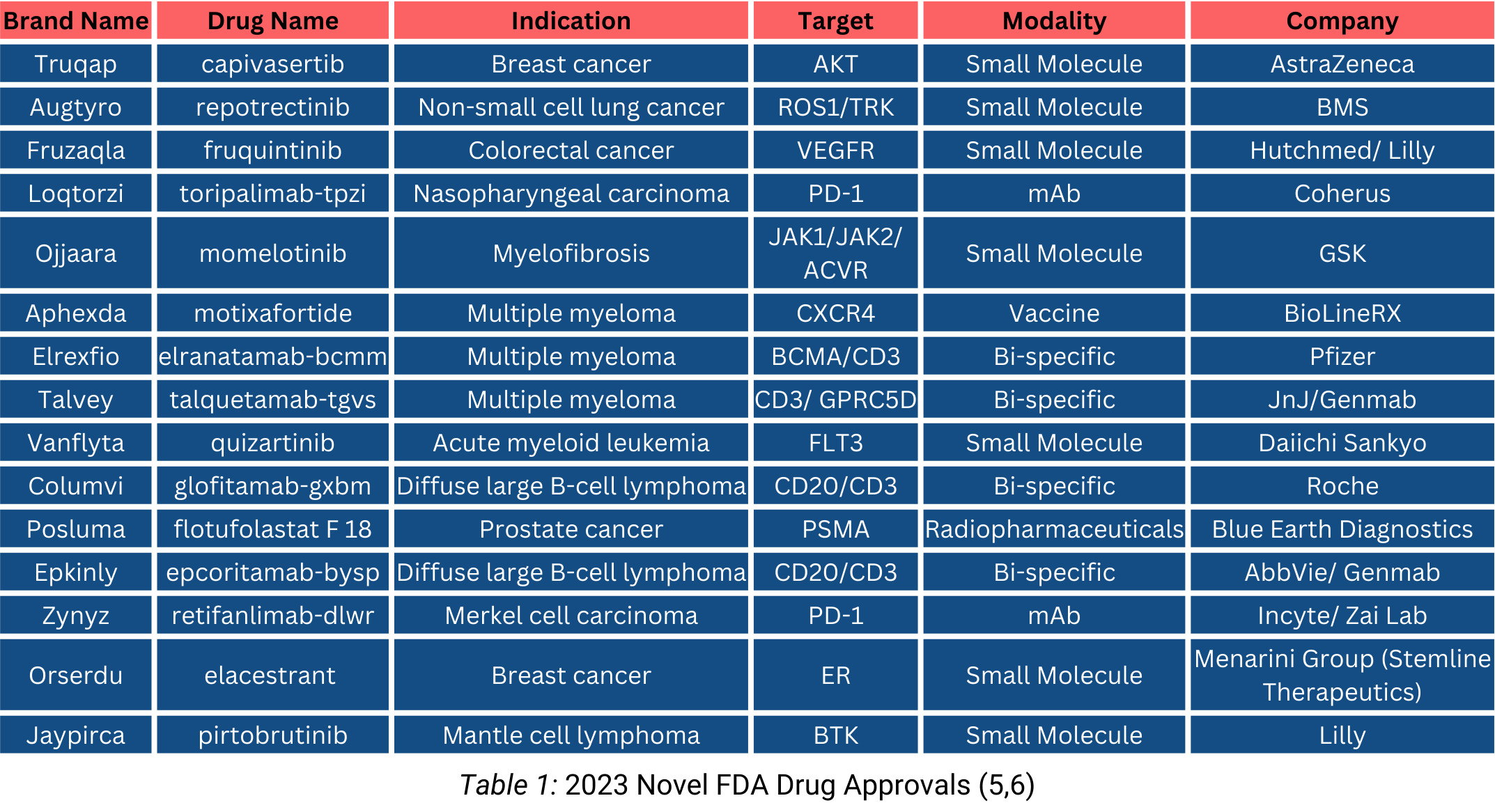
2023 FDA New Drug Approvals
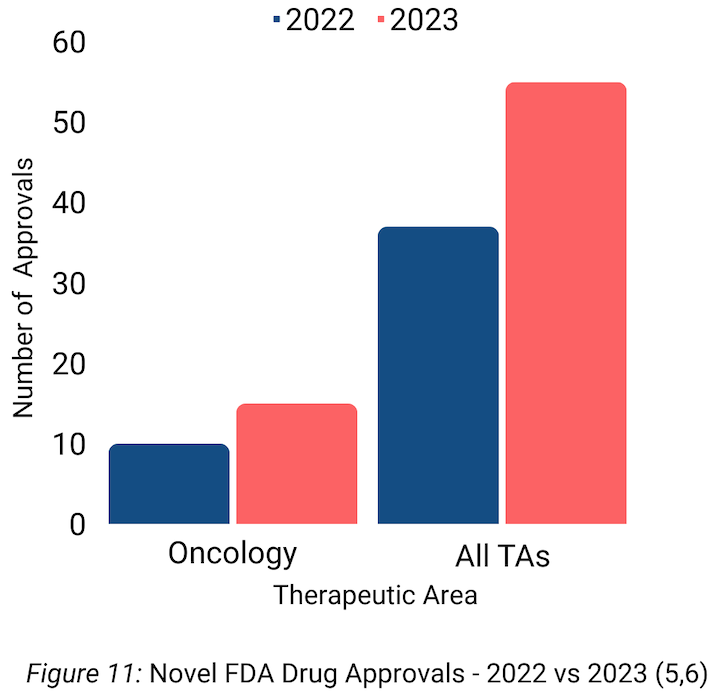
Continued innovation in the oncology space was demonstrated by a strong year for novel drug approvals. In 2023, the FDA approved 15 drugs for oncology indications (Table 1), up from 10 novel oncology approvals in 2022. Small molecules led the way in the oncology 40 space, accounting for 7 novel approvals in 2023. (5,6)
Similarly, total FDA novel drug approvals across all therapeutic areas were up nearly 50% with 55 in 2023 compared to 37 in 2022 (Figure 11).
Conclusion
Our analysis of oncology clinical trials showed that gastrointestinal cancers persisted as the most common indication. The surge in gastrointestinal cancer trials has paralleled an increase in cases of this indication. Lung cancer remains the second largest focus by trial volume. PD-1 remained a primary molecular target in checkpoint inhibitor exploration, with novel antigens like CLDN18.2, GPRC5D, and KRAS gaining interest. Additionally, promising precision medicine targets, such as DGK, emerged onto the scene.
Antibody-drug conjugates (ADCs) continued their momentum, with nearly 300 new trials in 2023. Seagen (now part of Pfizer) led the was in ADC development, predominantly employing MMAE payloads. Further, HER2 persisted as the most common ADC target.
Beyond ADCs, small molecules accounted for the largest proportion of the 15 oncology therapies garnering FDA approval in 2023, reflecting a productive year of innovation.
Overall, although small molecules and checkpoint inhibitors persist, momentum builds around targeted therapies exemplified by ADCs and novel antigens, underscoring the push towards more tailored and personalized cancer treatment approaches.
Sources
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8630546/
- https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660
- https://www.obroncology.com/leading-thoughts/top-lung-cancer-news-from-2023-and-whats-in-store- for-2024
- https://www.lungevity.org/blogs/2023-asco-highlights-of-lung-cancer-research
- https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic- biological-products/novel-drug-approvals-2023
- https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic- biological-products/novel-drug-approvals-2022
For more information, email info@kognitic.com
Copyright© 2023 Kognitic, inc. All rights reserved.*